Essential Guide to Finding the Limiting Reactant in 2025: Master Chemical Reactions!
Essential Guide to Finding the Limiting Reactant in 2025: Master Chemical Reactions!
Understanding Limiting Reactants
In the realm of chemical reactions, one of the key concepts to grasp is the idea of the limiting reactant. A limiting reactant is the substance that is completely consumed during a chemical reaction, thus determining the amount of product formed. In basic terms, it is the reactant that limits the extent of the reaction, and its identification is crucial for accurate stoichiometry. Thorough comprehension of how to determine limiting reactants is essential for both students and professionals working in advanced chemistry.
The Importance of Identifying Limiting Reactants
Identifying the limiting reactant is critical because it not only affects the theoretical yield but also the efficiency of the reaction. For example, in the reaction between hydrogen and oxygen to produce water, if hydrogen is the limiting reactant, any excess oxygen will remain unreacted. Such scenarios highlight the importance of understanding reactant quantities and their roles in reaction stoichiometry.
Steps to Identify the Limiting Reactant
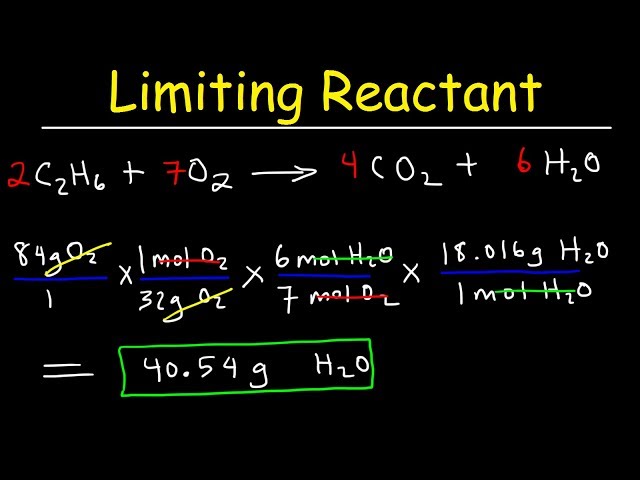
To find the limiting reactant, follow these systematic steps: First, write and balance the chemical equation for the reaction at hand. Second, determine the number of moles of each reactant you have available. Next, use the stoichiometric coefficients from the balanced equation to find out how many moles of product can be produced from each reactant. The reactant that produces the least amount of product is the limiting reactant. This basic approach allows for practical applications in laboratory techniques, aiding in accurate yield calculations.
Applying Stoichiometry to Calculate Limiting Reactants
Stoichiometry plays a significant role in chemical reactions. It allows chemists to calculate how much product will be generated from given quantities of reactants. The mole ratios derived from balanced equations are key to this. By utilizing these ratios, one can effectively calculate limiting reactants and understand how reactant ratios impact overall reaction efficiency.
Calculating Moles for Reaction Efficiently
To perform stoichiometric calculations accurately, you’ll need to understand how to convert mass to moles. Start by measuring the mass of your reactants and then convert this mass into moles using their respective molar mass. This conversion is a critical aspect of a successful stoichiometric analysis. Typically, the formula used is: moles = mass (g) / molar mass (g/mol), where you insert values for each reactant for precise calculations.
Factors Influencing Limiting Reactants
In addition to calculations, it’s essential to recognize the factors affecting which reactant becomes limiting. Environmental conditions, such as temperature and pressure, also play a crucial role. For instance, in gas reactions, higher pressures may lead to variations in stoichiometric ratios, thus shifting the limiting reactant status. Understanding these factors adds depth to your analysis and enhances chemical reaction efficiency.
Common Laboratory Techniques for Identifying Limiting Reactants
Laboratories utilize various techniques to accurately identify limiting reagents. Among these are gravimetric analysis and titration methods. Both approaches help chemists understand the completion of reactions and the role of each reactant, ensuring that the yield calculations reflect true reaction dynamics.
Gravimetric Analysis Explained
Gravimetric analysis involves measuring the mass of a reactant or product to determine the concentration of reactants in a given reaction. This method is particularly useful in cases where the reaction completion is visually resolvable, allowing for effective measurement of mass conservation throughout the reaction. By analyzing precipitates formed or the remaining reactants, one can calculate the limiting factor accurately.
Titration Techniques for Limit Reactions
Titration methods provide an interactive way to examine the relationship between reactants. By adding a titrant to a solution—and detecting the equivalence point—chemists can see how much reactant is needed for complete reaction, thereby identifying whether a reactant is excess or limiting. Understanding concentration calculations really opens the door to precision in analytical chemistry practices, enhancing your grasp on various chemical syntheses.
Advanced Stoichiometry: Theoretical vs. Practical Yield
Understanding the difference between theoretical yield and practical yield is essential for grasping the concept of limiting reactants. Theoretical yield is the amount of product predicted based on stoichiometric calculations, whereas practical yield is what is actually obtained from a reaction. Knowing this distinction is pivotal for evaluating chemical reaction efficiency and making informed predictions about reaction outcomes.
Calculating Theoretical Yield
Theoretical yield can be calculated by taking the limiting reactant and utilizing its moles, converting these into the product using stoichiometric ratios derived from the balanced chemical equations. For example, if you know the limiting reactant can produce a certain amount based on moles, you can multiply to find the maximum expected product, thus determining theoretical yield accurately.
Comparing Yields: Insights and Implications
Moreover, it’s crucial to compare theoretical yield with practical yield to understand any discrepancies. Understanding these differences can provide valuable insights on aspects such as reaction completion or whether there were any unaccounted losses in the process. This is essential in refining your techniques within practical chemistry and can point out areas needing improvement.
Key Takeaways
- Identifying the limiting reactant is fundamental for predicting reaction outcomes.
- Utilizing stoichiometric calculations is vital for accurate yield calculations.
- Understanding differences between theoretical and practical yields helps evaluate reaction efficiency.
- Common laboratory methods such as gravimetric analysis and titration techniques enhance precision in experiments.
- A firm grasp of stoichiometry is essential in mastering chemical reaction dynamics.
FAQ
1. What is a limiting reactant in a chemical reaction?
A limiting reactant is the substance that is used up first in a chemical reaction, limiting the amount of product that can be formed. Understanding this concept is fundamental in chemical calculations.
2. How do I find the excess reactant after identifying the limiting reactant?
To determine the excess reactant, first calculate how much of each reactant is consumed based on the balanced chemical equations. Subtract the amount used from the initial quantities to find the remaining excess reactant that did not participate in the reaction.
3. What are some common examples of limiting reactants in laboratory experiments?
In practice, a common example would be the reaction between hydrochloric acid and sodium bicarbonate, where usually the acid is the limiting reactant. This understanding improves your grasp of stoichiometric calculations.
4. How does stoichiometry relate to yield calculations?
Stoichiometry allows for the conversion of mass and moles in a reaction, helping you predict theoretical yields easily, based on the limiting reactant. It provides an essential foundation for understanding how reactants convert into products efficiently.
5. Why is it important to balance chemical equations?
Balancing chemical equations is critical for applying stoichiometric principles accurately. It ensures that you have the correct molar ratios needed for calculating reactant quantities and predicting reaction outcomes accurately.
6. Can reaction conditions affect which reactant is limiting?
Absolutely, reaction conditions such as temperature, pressure, and concentration can all impact the relative amounts of reactants and hence which one becomes limiting. Recognizing these factors is crucial for predictive chemical methodologies.
7. What techniques can help with stoichiometric calculations?
Taking advantage of tools such as the mole map and being familiar with conversion techniques like mass to mole conversions can significantly enhance your efficiency in stoichiometric calculations, ensuring more accurate laboratory techniques.

