Effective Ways to Calculate Moles in 2025: Discover the Best Techniques!
Effective Ways to Calculate Moles in 2025
Understanding how to calculate moles is fundamental for anyone studying chemistry, as it helps to quantify the amount of substance in various chemical reactions and scenarios. With a clear grasp of mole calculations, students and professionals alike can navigate problems with ease. In this article, we’ll explore essential methods to calculate moles, provide practical examples, and delve into the importance of moles in chemistry.
Understanding Moles in Chemistry
The concept of moles is critical in chemistry, acting as a bridge between the atomic and macroscopic worlds. A mole represents \(6.022 \times 10^{23}\) entities, such as atoms or molecules, known as Avogadro’s number. The beauty of understanding chemical moles lies in its ability to simplify calculations involving molar mass, moles in reactions, and stoichiometry.
What is a Mole?
A mole is a unit of measurement used to express amounts of a chemical substance. It provides a method for chemists to count particles at the atomic level. For instance, one mole of carbon contains exactly \(6.022 \times 10^{23}\) atoms. This definition is critical for learning how to find moles, especially when translating between the mass of substances and the number of moles present.
The Role of Molar Mass
Molar mass is pivotal in mole calculations. It is defined as the mass of one mole of a substance, expressed in grams per mole (g/mol). For example, the molar mass of water (H₂O) is approximately 18.02 g/mol. This means if you have 36.04 grams of water, you have exactly 2 moles. By understanding how to calculate and utilize molar mass, you can efficiently convert between grams and moles, ensuring accurate outcomes in calculations.
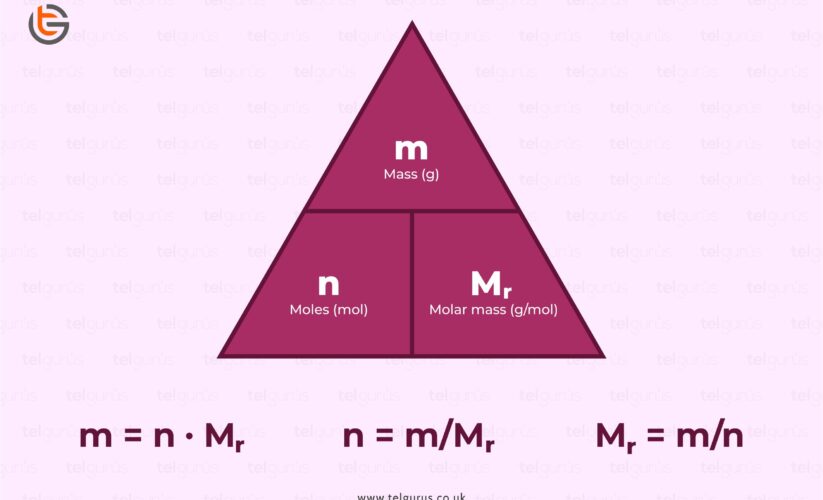
Calculating Moles from Mass
To determine the number of moles from a known mass, you can use the formula:
\[ \text{Number of Moles} = \frac{\text{mass (g)}}{\text{molar mass (g/mol)}} \].
For example, if you have 50 grams of sodium chloride (NaCl) with a molar mass of approximately 58.44 g/mol, the calculation would be:
\[ \text{Number of Moles} = \frac{50\, g}{58.44\, g/mol} \approx 0.856\, moles \]. This method illustrates the relationship between mass and moles, helping to translate weights into mole units seamlessly.
Mole Conversion Techniques
Conversion between grams, liters, and moles is a fundamental skill in chemistry. Knowing how to perform mole conversions effectively can be the key to solving various problems encountered in chemical equations and experimental calculations.
Grams to Moles Conversion
Converting grams to moles is essential for utilizing stoichiometry in chemical reactions. The primary formula for this conversion is the same as previously mentioned. For instance, if you want to determine how many moles are in 200 grams of ammonium sulfate (NH₄)₂SO₄, you start with its molar mass, around 132.14 g/mol:
\[ \text{Number of Moles} = \frac{200\, g}{132.14\, g/mol} \approx 1.51\, moles \]. By repeating this process with different substances, you’ll grow comfortable making conversion to moles.
Moles to Liters Conversion
When dealing with gases, it’s often useful to convert moles to liters. This is based on the Ideal Gas Law, which posits that at standard temperature and pressure (STP), one mole of an ideal gas occupies approximately 22.4 liters. Therefore, if you know the number of moles, you can simply multiply by this volume. For example, 3 moles of gas can be converted to:
\[ 3\, \text{moles} \times 22.4\, \text{L/mole} = 67.2\, \text{L} \]. This highlights the practical application of moles in real-world scenarios.
Moles in Solutions: Understanding Molarity
Molarity is another vital concept tied closely with moles. Defined as moles of solute per liter of solution, the molarity formula is:
\[ \text{Molarity (M)} = \frac{\text{moles of solute}}{\text{liters of solution}} \]. Say you dissolve 2 moles of sodium chloride in 1 liter of water, you would say that the solution has a molarity of 2 M. This relationship between molarity and moles in solutions allows chemists to quantify concentration and perform effective dilutions in laboratory settings.
Applying Moles in Chemical Reactions
Mole ratios in balanced chemical equations illustrate the relationships between reactants and products, making it crucial for any calculations involving stoichiometry. Understanding these ratios allows for accurate predictions of the amounts needed in reactions.
Using Mole Ratios
Mole ratios are derived from the coefficients of a balanced equation. For example, in the equation 2H₂ + O₂ → 2H₂O, the mole ratio of hydrogen to water is 2:2 or 1:1. If you start with 4 moles of hydrogen, you’d produce 4 moles of water using this ratio. Knowing these relationships is invaluable when determining reactant amounts and ensuring the proper stoichiometric balance.
Limiting and Excess Reactants
In many reactions, one reactant is used up before the others; this reactant is known as the limiting reactant. Understanding how to calculate reactant moles and determine excess moles is crucial for optimizing reactions. If you start with 10 moles of A and 4 moles of B in a reaction A + B → C, knowing the mole ratio will help you discern which reactant will limit the production of C and predict leftover moles from the other reactant more accurately.
Practical Example of Moles in Reactions
Let’s consider a practical scenario. In a reaction involving 2 moles of hydrogen gas reacting with oxygen, you produce water. By applying the mole ratios, if you originally have 5 moles of hydrogen and excess oxygen, you would form 5 moles of water, using only 5 moles out of the required 2 (because 5 is the maximum allowed by the balanced equation). This illustrates real-world application in chemical stoichiometry.
FAQ
1. How do I calculate molarity from moles of solute?
To calculate molarity from moles of solute, use the formula M = moles of solute/liters of solution. For example, if you dissolve 0.5 moles of NaCl in 0.25 liters of water, the molarity would be 0.5/0.25 = 2 M.
2. What are mole ratios?
Mole ratios express the relationship between reactants and products in a balanced chemical equation, allowing chemists to predict how much of a product will form based on the quantities of reactants available.
3. How can I convert grams to moles for different substances?
To convert grams to moles, divide the mass of the substance in grams by its molar mass (g/mol). Ensure that you know the molar mass of the substance you are calculating for accurate conversions.
4. Why is understanding moles important in chemistry?
Understanding moles in chemistry is crucial for quantifying reactions, determining concentrations, and performing accurate calculations that are foundational in laboratory and theoretical chemistry.
5. Can you give an example of using moles in a chemical equation?
Sure! If you have the chemical equation 2H₂ + O₂ → 2H₂O, this means that you need 2 moles of hydrogen for every 1 mole of oxygen to produce 2 moles of water, illustrating important mole usage in reactions.
6. What are grams per mole?
Grams per mole (g/mol) denote the molar mass of a substance, indicating how many grams correspond to one mole, thus serving as a conversion factor in mole calculations.
7. How do I find the number of moles in a gas?
To find the number of moles in a gas, you can apply the ideal gas law: PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature. Utilizing these variables can help estimate moles accurately.
Chemistry is significantly enriched by mastering the concept of moles. As we advance deeper into related topics, leveraging the foundational principles outlined here will elevate your understanding and proficiency. Whether solving equations or performing lab experiments, knowing how to accurately calculate moles is a skill that will serve you well in the field of science.